PUFAs and Seed Oils: Why These Fats Damage Your Metabolism and Overall Health
This post is a PUFA deep dive. You’ll learn what they are, how they affect your health, and how to avoid them, so you can feel empowered to make better choices for yourself and your family.
------------------------------------
Over the last century, our food system has changed in ways most people don’t even realize, but nearly everyone feels.
The very fatty acid composition of our diet has shifted, reshaping how we fuel our bodies and how our metabolism functions.
Polyunsaturated fatty acids (PUFAs), once a minor part of the human diet, have become a major player. Why? Because of the rise of industrial seed oils like soybean, corn, and canola oil — plus the shift in how we raise livestock. Conventional chicken and pork, for example, come from animals kept indoors and fed high‑PUFA diets made possible through industrial agriculture after WWII.
Most people think of seed oils when they hear PUFAs, but these fats are hiding in far more places than your frying oil.
PUFA-rich source in modern diets:
- - Seed oils (soybean oil, corn oil, canola oil, grapeseed oil, rice bran oil, sunflower oil & more)
- - Conventional chicken and pork (especially fatty cuts like chicken wings, drumsticks, thighs, bacon, ground pork, sausage & more)
- - Excess nut, seed and nut-butter consumption
- - Dressings and condiments made with seed oils
- - Fried food, and most restaurant food
For decades, PUFAs have been marketed as “heart‑healthy.” But research increasingly suggests a different story: these fats are unstable, prone to oxidation, can interfere with energy production, and even slow down your metabolism.
And we’re eating far more of them today than our ancestors ever did.
Consider this: soybeans, a major source of PUFAs, weren’t even part of the American diet until the early 1900s. Back then, soybean oil was used to make paint. (r) Today, soy dominates both our food system and livestock feed, exposing us to more PUFAs than ever before.
Table of Contents:
- Intro
- What are PUFAs
- The Big Fat Shift
- Why PUFAs are harmful to our health
- PUFAs vs Saturated Fats
- Aren't PUFAs essential?
- What to do
- The LowPs™ Difference at Nourish Food Club

Note: This is not medical advice. This blog post is for informational and educational purposes only.
Introduction
In this blog we will be exploring how the modern food system, shaped by decades of misinformation and Big Ag influence, has lowered our metabolic rates by changing the kinds of fats in our diets.
But first, let’s set the stage with a big-picture perspective:
The types of fat you eat play a much bigger role in your weight, energy, and overall health than you realize.
Dietary fats directly influence how your body produces energy, whether you burn or store fat, and how well you metabolize carbohydrates.
In other words, the fats you choose help shape your metabolism.
“Metabolism” is a popular buzzword these days, but at its core, it simply refers to how your body transforms the food and drink you consume into energy and the raw materials needed for survival. It’s a vital process, yet many aspects of modern life interfere with it working as it should.
Your metabolism determines how many calories your body burns at rest (which affects body fat levels) and how much cellular energy (ATP) you produce, fueling everything from digestion and heartbeat to brain function, hair growth, tissue repair, and physical activity.
A higher metabolic rate makes it easier to maintain a healthy weight and sustain the energy needed for optimal function.
Conversely, a downregulated metabolism means you need fewer calories just to maintain your weight (making weight loss harder), and many bodily functions slow because there’s less cellular energy (ATP) to go around.
When this happens, something has to give.
Vital processes like keeping your heart beating are prioritized, while non-essential tasks are dialed down, often showing up as frustrating symptoms. The body is doing the best it can given the resources (energy) it has.
With this foundation in mind, we can now dive into PUFAs: what they are, why they’ve become so common in our food system, and how the types of fats we eat can make or break a healthy metabolism.
What are PUFAs?
Fats are a group of chemical compounds made up of fatty acids, the building blocks of the fat in our bodies and in the food we eat. At the molecular level, different types of fatty acids have unique structural properties, which influence how they behave in the body:
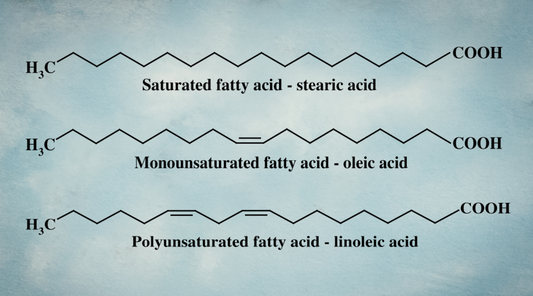
- - Saturated Fatty Acids (SFA): Contain only single bonds between carbon atoms, making them highly stable and resistant to oxidation. Examples include stearic acid, palmitic acid, C15:0, and lauric acid.
- - Monounsaturated Fatty Acids (MUFA): Contain one double bond, making them slightly more reactive than SFAs but still relatively stable. Olive oil (high in oleic acid) is a well-known MUFA source.
- - Polyunsaturated Fatty Acids (PUFA): Contain two or more double bonds, making them far more prone to oxidation. This category includes both omega-6s (like linoleic acid, found in seed oils) and omega-3s (like DHA and EPA, found in fish).

Why do double bonds matter?
A single bond shares one pair of electrons between two atoms (shown as a single line in biochemical diagrams). While weaker than double or triple bonds, single bonds are more stable and less reactive, which is why saturated fats are far less likely to oxidize.
In contrast, a double bond shares two pairs of electrons (shown as two lines between atoms, highlighted by red arrows above). These bonds create “kinks” in the molecule, making fats with double bonds, like PUFAs, less stable and more prone to oxidation (r,r).
When exposed to heat, light, or oxygen, PUFAs lose electrons, leading to free radical damage, inflammation, and disruptions in cellular signaling: all of which can ultimately impair metabolism and overall health.
All dietary fat sources are a combination of these different types of fats, but in different ratios.
For example, butter and cheese contain a much higher proportion of saturated fat and a more favorable saturated-to-unsaturated fat ratio compared to margarine, which is made from seed oils and loaded with PUFAs.

While both may contain similar amounts of total fat per tablespoon, their composition determines how they impact cellular health, metabolic signaling, and overall function in the body.
Now that we understand the different types of fats, let’s discuss how and why the fat composition of our diets has drastically changed.
The Big Fat Shift
Over the past century, unsaturated fat consumption has skyrocketed far beyond historical norms, both in the fats we cook with and in the animal products we eat.
Traditional fats that sustained generations like butter, dairy and tallow (higher in saturated fats) have been pushed aside in favor of highly processed seed oils, plant based dairy products, and margarine (all higher in plant-based PUFAs like linoleic acid, LA, and alpha-linolenic acid, ALA).
At the same time, we’ve shifted what livestock are fed: corn oil, soy, vegetable oils, and dried distillers grains, all high in plant-based PUFAs as well.
And as a result, conventional pork, chicken, turkey, and eggs contain more PUFA levels than ever before. (r,r)
Scientists and food producers have even shown that the fatty acid composition of milk and dairy can be manipulated simply by changing what cows and goats are fed. (r)
In other words: what animals eat directly changes the fats we get from them.
One study put it plainly:
“Saturated FA levels in [chicken fat tissue] decreased significantly…with an increase in dietary PUFA…. The concentration of linoleic acid increased significantly with an increase in dietary PUFA.” (r)
Feeding livestock high-PUFA diets has shifted the fat profile of the animal products we eat: giving us more PUFAs and less metabolism-supporting saturated fat.
This means that even if you’re avoiding seed oils and skipping PUFA‑rich plant‑based dairy alternatives, you’re still likely getting more of these metabolism‑suppressing PUFAs hidden in the fat of most modern meat, dairy, and eggs.
We’re not just changing what we eat…we’re changing the nutritional makeup of our entire food chain.
It wasn’t always this way.
Before 1900, PUFAs made up just 1–2% of daily caloric intake. Today, they often account for 15% or more, a five- to six-fold increase. This rise is strongly linked to metabolic dysfunction, chronic inflammation, and disease.
So how did this big “fat shift” happen?
- - Big Ag, chemical-based agriculture, and government subsidies led to the over production of cheap seed oils.
- - ‘Heart Healthy’ marketing demonized animal fats and cholesterol, to sell you more statins and pharmaceuticals, many of this misinformation has now been disproven (r).
-
- Health agencies (heavily influenced by industry) have demonized animal fats and promoted plant-based fats, rich in LA and ALA, fats our ancestors never consumed in large amounts.
This shift was built on decades of misinformation, rooted in a misplaced fear of saturated fat and cholesterol.
And how has it turned out for us?
Not well.
-- Obesity rates and chronic diseases, including cardiovascular disease and Type 2 diabetes, are on the rise (r).
-- Life expectancy is declining. (r)
-- A 2022 study (r) found that 93% of Americans are not metabolically healthy, and over one-third of U.S. adults have prediabetes.
The signal is clear: our metabolisms are not functioning the way they should.
Thankfully, mounting evidence is challenging these outdated, PUFA-rich and saturated fat fear mongering dietary recommendations (r).
> A recent meta-analysis by Ramsden found that LA intake was correlated with an increased risk of cardiovascular events and all-cause mortality (r), contradicting the mainstream “heart-healthy” narrative that has been widely endorsed.
> Experts are beginning to acknowledge the lack of long-term, large-scale human studies on the impact of higher PUFA intake (r), raising even more doubts about the safety of these fats.
> New research continues to dismantle the cholesterol myth (r), revealing that oxidized linoleic acid, not cholesterol itself, is the real driver of heart disease. (r,r)
> While replacing saturated fat with PUFA-rich seed oils has been shown to lower serum cholesterol, recent studies do not support the idea that replacing saturated fat translates to a lower risk of heart disease or overall mortality (r) turning the long-held belief that “saturated fat is bad for you” on its head.
> And a new 2025 meta-analysis of randomized controlled trials (RCTs) concluded: “The recommendation to limit dietary saturated fat intake is primarily drawn from observational studies rather than randomized controlled trials of cardiovascular disease prevention…the evidence available from RCTs does not support saturated fat restriction for the prevention of cardiovascular disease.” (ref)
So when will mainstream nutrition admit it?
It’s long past time to question whether these industry-shaped dietary recommendations (so different from what our ancestors consumed) are working.
Why are PUFAs Harmful to Health in Excess?
The dramatic shift in the types of fats we consume isn’t just a minor dietary change… it’s a biological disruption.
These changes alter how our bodies produce energy, and since energy production fuels every cell and system, the ripple effects are felt everywhere.
This dietary fat shift has had massive consequences for human health, including:
-- Lowered metabolism
-- Disrupted energy production
-- Increased risk of overweight and obesity
-- Greater insulin resistance and impaired carbohydrate utilization
-- Higher rates of oxidative damage throughout the body
-- Increased cardiovascular disease risk
-- Disrupted gut health
-- Increased physiological stress
-- Hormonal imbalances
-- Changes in your own fat stores, altering their structure and function

While many of these effects are interconnected, it’s worth examining them individually to see how down-regulated metabolism impacts the entire organism.
1. Lower Metabolism
The types of fatty acids you consume (which ultimately become part of your body’s own fat stores) play a huge role in the “calories out” side of the energy balance equation.
Energy balance refers to the relationship between calories consumed (“calories in”) and calories expended (“calories out”).
- - Calories in: All the food and caloric drinks you consume
- - Calories out: All the energy your body uses each day, from physical activity to digestion, but most importantly your basal metabolic rate (BMR)
Your BMR is the energy required to maintain basic functions at rest, like breathing, cell repair, and keeping your organs running. It actually accounts for the largest share of daily energy expenditure because every single function in your body requires energy.
When calories in exceed calories out, weight is gained. When calories out exceed calories in, weight is lost. And when they’re balanced, weight is maintained.

The Big Problem: our ‘Calories Out’ has declined.
What many people don’t realize is that our basal metabolic rate (BMR), the biggest component of “calories out”, has dropped significantly over time. This reduction in “calories out” slows energy production in your cells, making fat gain and metabolic dysfunction far more likely.
Dr. John Speakman, a leading metabolism researcher, confirmed this decline (r):
- - Men now burn about 220 fewer calories per day at rest compared to past generations.
- - Women burn about 122 fewer calories per day.
This decrease happened despite increases in physical activity, meaning the reduction in resting metabolism is the real driver.
Speakman concluded: “The magnitude of this effect is sufficient to explain the obesity epidemic”

Why is this happening?
One major culprit: the shift in dietary fat quality: away from saturated fats and toward polyunsaturated fats (PUFAs).
Research consistently shows that PUFAs lower metabolic rate (r,r,r,r,r,r,r,r), causing your body to burn fewer calories at rest, which means you can gain weight more easily, even without increasing your food intake.
Numerous animal studies reveal the profound metabolic impact of changing fat intake.
Rats fed diets low in PUFAs and high in saturated fat (like hydrogenated coconut oil) experienced a significant increase in basal respiration per unit of body weight. (r) Cytochrome oxidase activity, a key mitochondrial enzyme in energy production, was 38% higher in the low-PUFA group. Plus, the low PUFA fed rats had 21-25% lower body weight after 9-12 weeks, despite equivalent calorie intake.
In another rat study, resting metabolism in relation to body surface area was increased by 25% when sunflower oil (high in PUFA) was subbed for coconut oil (higher in saturated fat). (r)
The takeaway?
The higher the percentage of saturated fat in the diet, and the lower the percentage of unsaturated fat, the higher the metabolic rate.
Dr. Speakman’s work echoes this: (r)
“We varied the fatty acid composition of mouse diets and showed that their energy expenditure (adjusted for body composition) increased as their consumption of saturated fat increased. This raises the interesting possibility that reducing saturated fat consumption may have driven reductions in BEE (basal energy expenditure).”

This also ties into your thyroid, the master regulator of metabolism.
This is supported by what happens with your thyroid system based on different dietary fats.
PUFAs interfere with thyroid function at multiple levels: suppress the thyroid gland itself, inhibit enzymes that convert T4 into T3, and interfere with how T3 binds to transport proteins and receptors. All of these blockages prevent you from getting active T3 thyroid hormone to your cells (r,r,r,r,r,r,r) which in turn slows the metabolic rate.
The big picture: the saturated-to-unsaturated fat ratio in the American diet has drastically shifted toward PUFAs, especially omega‑6 fats like linoleic acid.
Generations before us consumed fewer PUFAs, more saturated fats, and maintained leaner, healthier metabolisms. Today, with PUFAs making up a greater percentage of our diet than ever, we’re facing an obesity and metabolic crisis.
2. Disrupts Energy Production
Energy production is a core part of metabolism: it’s the process of converting the nutrients you eat (carbs, fats and protein) into ATP, the usable energy for your cells.
The more energy you produce, the better your body functions. Period.
When energy production fails, tissues can’t maintain their proper structure or function, and symptoms often appear first in the most vulnerable organs. Downregulated metabolism lowers energy output as a survival mechanism, but it comes at the cost of optimal health.
To understand how dietary fats influence this, we need to look deeper at how they affect energy production at the cellular level.
Saturated and unsaturated fats have different structures, so of course your body processes them differently.
And when it comes to energy production, higher‑PUFA diets can create significant disruptions.
Why?
Theres a few levels to this.
First, it starts with oxygen availability.
To stay healthy and maintain a healthy body weight, you need a strong metabolism. Meaning, you efficiently burn the food you eat and produce sufficient energy.
This process depends heavily on oxygen availability at the cellular level.
High-PUFA diets can interfere with this by using up valuable oxygen.
Your body uses up valuable oxygen to process, manage, detox and eliminate excess PUFAs (ref, ref), leaving less oxygen available for optimal energy production.
As a result, your body shifts away from oxidative phosphorylation (the ideal, oxygen-based energy pathway) toward glycolysis (a less efficient, back-up fermentation pathway).
In short: higher PUFA diets consume oxygen (ref), leaving less for robust ATP production through oxidative phosphorylation. This drives an upregulation of glycolysis, lowers energy efficiency, and ultimately suppresses metabolic rate.

The second piece here is that PUFAs and Saturated fats are burned differently during beta oxidation: the process your body uses to break down fatty acids (the building blocks of fats) into usable energy.
Your metabolism takes the food you eat and converts it into ATP — the usable energy for your cells. To do this, you need high‑energy electrons (that are present in your food) and molecules that carry them — think of these electron carriers (NADH and FADH2) as taxi cabs that shuttle electrons through the vital steps of ATP production.
Here’s the flow:
>> Fatty acids are long chains of carbons with hydrogens attached.
>> Through beta‑oxidation, these chains are “chopped” into two‑carbon units called acetyl‑CoA.
>> Acetyl‑CoA enters the citric acid cycle (TCA cycle): think of this as the prep stage. It doesn’t make much ATP directly but generates the vital electron carriers (NADH and FADH₂), the taxis that deliver electrons to the electron transport chain (ETC).
>> The ETC is the final step of energy production inside the mitochondria, where electrons move through a series of complexes, creating the proton gradient that drives ATP synthesis: the bulk of your energy output.
In short:
The carbon part of fat becomes acetyl‑CoA, fueling the TCA cycle to generate electron carriers.
The hydrogen part of fat provides the electrons, which are loaded into those carriers and delivered to the ETC: where most ATP is ultimately produced.
Because of their chemical structure, PUFAs generally yield less ATP per carbon than saturated fats and “burn dirtier”.

Let’s briefly dive into why.
In beta-oxidation, the fatty acid chains are progressively ‘chopped down’. Each round of beta-oxidation produces 1 NADH (removing hydrogens), 1 FADH2 (first dehydrogenation step), 1 acetyl-CoA (2-carbon unit).
Saturated fats have no double bonds, meaning every carbon is fully “saturated” with hydrogens. This allows them to move through a standard beta‑oxidation cycle at every step, consistently making NADH and FADH₂.
PUFAs, in contrast, have double bonds that replace some hydrogens.
This means:
- - There are fewer hydrogens to harvest (fewer electrons for NADH and FADH₂).
- - The beta‑oxidation pathway must use extra enzymes (enoyl‑CoA isomerase, 2,4‑dienoyl‑CoA reductase) to bypass double bonds. These detours skip some normal FADH₂‑producing steps, reducing total ATP output.
Saturated fats have no double bonds, so each carbon is fully “saturated” with hydrogens.
Thus, Saturated fats go through a standard beta‑oxidation cycle at every step, consistently making NADH + FADH₂.
With PUFAs, double bonds replace some of these hydrogens, so the beta‑oxidation pathway skips certain dehydrogenation steps (specifically the ones that normally produce FADH₂). Beta-oxidation of PUFAs requires additional enzymes (e.g., enoyl‑CoA isomerase, 2,4‑dienoyl‑CoA reductase) to bypass their double bonds. These detours skip some of the normal FADH₂‑producing reactions.
In short: Even though both saturated fats and PUFAs generate the same amount of acetyl‑CoA per carbon, PUFAs make fewer NADH and FADH₂ per carbon — meaning fewer electrons to fuel the ETC and ultimately less ATP per molecule of fat oxidized.
We want more FADH2!
And saturated fats (like stearic acid) generate more FADH2 per carbon than PUFAs.
This matters because FADH₂ feeds electrons into Complex II of the ETC, helping maintain a smooth electron flow and supporting a favorable redox state (a higher NAD⁺/NADH ratio).
Think of NAD⁺ as an empty taxi ready to pick up electrons and NADH as a full taxi. If too many taxis are full, traffic stalls and energy production slows. Saturated fats help keep more taxis empty and ready, keeping electrons moving efficiently through the ETC for cleaner energy production and more ATP output.
Plus, the higher FADH2/NADH ratio with Saturated fats creates more reverse electron transport (RET) at Complex 1, which produces more superoxide. While superoxide is a reactive oxygen species (ROS), this ROS isn’t bad! Instead, it acts as an important signaling mechanism that helps regulate metabolism and insulin sensitivity.
PUFAs generate fewer NADH and FADH₂ per carbon during beta‑oxidation because of their structure (fewer hydrogens + skipped steps).
Since these carriers are what actually fuel the ETC, PUFAs ultimately produce less ATP per carbon than saturated fats.
In contrast, saturated fats undergo straightforward beta‑oxidation, producing a full set of electron carriers (NADH and FADH₂) at each step. These carriers then feed electrons into the electron transport chain (ETC), where they generate a strong proton gradient to power ATP synthesis.
The extra enzymatic steps in PUFA beta‑oxidation not only lower ATP yield but also increase electron leakage in the mitochondria (r,r), leading to more oxidative stress.
This creates ROS in the wrong places (different than the superoxide convo above), damaging mitochondrial membranes and enzymes — further slowing energy production and contributing to metabolic dysfunction. PUFAs increase peroxidation (oxidation of the fats themselves), generating harmful lipid peroxides and downstream toxic byproducts (like 4‑HNE).
In fact, lowering PUFA intake has been shown to help restore mitochondrial function, including improving one of the final steps in the ETC (cytochrome c oxidase) (r).
So again, here’s the big picture:
- Saturated fats: Burn more “cleanly,” produce more FADH₂, support a healthier redox state, generate more superoxide (a beneficial metabolic signal), reduce oxidative stress, and fuel a higher metabolic rate.
- PUFAs: Burn less efficiently, generate fewer electron carriers, generate less superoxide, but increase oxidative stress through lipid peroxidation, and suppress metabolic function over time.
A higher saturated‑to‑unsaturated fat ratio supports a healthier metabolism, while high‑PUFA diets dampen it by interfering with mitochondrial energy production.
Understanding these differences is key to making dietary choices that support long‑term energy production and metabolic health.
3. Increase risk of Obesity
Due to the factors we’ve discussed above, it becomes easier to see why diets higher in unsaturated fats, especially omega‑6 PUFAs, can increase the risk of obesity.
Metabolism plays a central role in weight regulation by balancing how many calories you consume, burn, and store. A well‑functioning metabolism converts more of the energy you eat into ATP, making it easier to burn fuel instead of storing it.
This is why a higher metabolic rate allows you to maintain a healthy weight: you burn more calories at rest and can eat without storing excess fat.
On the flip side, a lower metabolic rate means you burn fewer calories at rest, making fat gain much more likely, even on lower‑calorie diets.
When metabolic signaling is disrupted, fewer calories are converted to ATP, more are stored, and fat cells grow larger.
PUFAs interrupt metabolic signaling.
Linoleic acid and other PUFAs generate less superoxide than saturated fats, which delays key satiety signals at the cellular level. This means more energy gets stored, less energy is produced, hunger lingers longer, satiety signals are never fired, and it becomes much easier to overeat (leading to fat gain).
PUFAs interfere with satiety and hunger signaling, making it easier to over eat.
This helps explain why so many people find it increasingly difficult to lose weight or maintain a healthy size, especially as metabolic health continues to decline across the population.
A lower metabolic rate means you burn less calories at rest and gain weight on lower calories.
Due to the downregulation of metabolism, research shows that excessive intake of linoleic acid and other omega-6 PUFAs can directly promote fat gain. In fact, this may be a major contributing factor to the obesity and diabetes epidemics. (r,r,r,r,r,r,r,r,r,r)
In one study, high serum DGLA levels (a metabolite derived from dietary linoleic acid) were associated with obesity, body fat accumulation, a high ALT level, and insulin resistance in Japanese patients with type 2 diabetes. (r)
Another study in a Spanish population found that adipose tissue LA content, closely tied to dietary LA intake, positively correlated with overall obesity and central fat accumulation. (r)


Animal Studies: Same Calories, Different Fat, Different Weight
Animal studies provide a clearer picture because they allow for strict control of variables like calorie intake and fat type, factors that are much harder to manage in human studies. They can also be extended over longer periods, offering more meaningful insights into how different fats impact metabolism and weight gain over time.
For example, in one experiment, mice on a low PUFA diet lost more weight (left chart) since their metabolic rate (right chart) was higher on equal calorie intake. (r) Their metabolic rate was significantly higher, and their mitochondria accessed more oxygen, enabling better energy production.

Here are some other striking findings from controlled animal studies:
“Our results indicate that, contrary to expectation, PUFA-rich soybean oil is more obesogenic and diabetogenic than coconut oil which consists of primarily saturated fat….While this study was in progress, two groups published papers with results similar to ours—namely, that a high fat diet supplemented with oils high in LA leads to obesity and fatty liver [24,53,146]. Other studies have also shown that dietary LA can cause adiposity in humans [165,166] and lead to hyperglycemia as well as obesity in mice [19,167].” (r)
“These results indicate that enrichment of the diet with polyunsaturated fatty acids causes changes in adipose tissue metabolism that favour fat deposition. Different metabolic pathways were preferentially affected by each type of fatty acid used.” (ref)
“a diet high in soybean oil is more detrimental to metabolic health than a diet high in fructose or coconut oil.” (r)
“These results suggest that an excessive intake of [omega-6 polyunsaturated fatty acids] is associated with obesity and its related metabolic abnormalities.” (ref)
“These data indicate that in male mice, LA induces obesity and insulin resistance, and reduces activity, more than saturated fat, supporting the hypothesis that increased LA intake may be a contributor to the obesity epidemic.” (ref)
“Despite isocaloric feeding, weight gain was lower in rats fed the more highly saturated ET-L diet than in those fed either the high (n-9) fatty acid OL-L diet or the high (n-6) fatty acid SAF-L diet.” (ref)
In this study, rats were fed standardized, isocaloric diets that differed only in their fat source: edible tallow (rich in saturated fat), olive oil (rich in monounsaturated fat), or safflower oil (rich in linoleic acid, an omega-6 polyunsaturated fat). Despite all groups consuming the same number of calories, the results showed significant differences in weight gain based on the type of fat consumed. Rats fed the safflower oil diet gained approximately 16% more weight than those fed olive oil, and about 36% more than those fed tallow (higher in saturated fat).
The striking differences in these studies suggests that the type of fat, not just the number of calories, can influence how the body stores energy and gains weight.




4. Impair Carb Metabolism
Insulin resistance isn’t caused by carbohydrates. It’s caused by an inability to properly use carbohydrates, and one major factor in that dysfunction is PUFAs.
PUFAs hinder your body’s ability to burn carbs. But before diving into why this happens, what does ‘hinder your body’s ability to burn carbs’ actually mean? PUFAs get in the way of your body taking the carbs you consume in your diet and converting it into ATP.
In short: poor metabolic flexibility, elevated blood sugar, insulin resistance, reduced energy production, and increased fat storage.
PUFAs disrupt the body’s ability to burn glucose efficiently by inhibiting enzymes essential for breaking down carbs (r,r). The result? Increased fat storage, insulin resistance (ref, ref, r), and a loss of metabolic flexibility (the ability to switch between burning carbs and fat, which is a natural process and something our bodies should be able to do).
Let’s break down how this works a little more.
Metabolic flexibility: carbs first, then fat.
In fact, there’s a forgotten phrase from the old-school medical literature:
“Fat burns in the flames of carbohydrates”
In a healthy metabolism, after eating a mixed meal (with both carbs and fat), your body is designed to burn carbohydrates first, then shift to fat burning as glucose is used up.
Here’s how it should work:
1. Post-meal: Blood sugar rises, insulin is released, and glucose becomes the primary energy source. Dietary fat is temporarily stored.
2. As insulin drops: Your body switches back to fat burning, using stored fat for energy.
This natural rhythm allows your body to smoothly alternate between fuel sources — a key marker of metabolic health called metabolic flexibility.
But PUFA consumption disrupts this entire process.
Here’s a twist that many don't realize: your ability to burn fat well can actually improve by properly burning carbs.
When the body burns fat, fatty acids are converted into acetyl-CoA, but to enter the citric acid cycle (the next step in energy production), acetyl-CoA needs oxaloacetate (r), which comes primarily from carbohydrate metabolism via pyruvate.
Without enough carbs:
-- Oxaloacetate levels can get depleted (especially if it’s being pulled for gluconeogenesis)
-- The citric acid cycle slows (lowering metabolic rate)
-- Fat metabolism stalls
This is why pyruvate is so important: it fuels the citric acid cycle, allowing both carbs and fat to be burned efficiently.
PUFAs, by impairing carbohydrate metabolism and pyruvate utilization, therefore disrupt fat metabolism too.
Hence the saying: “Fat burns in the flame of carbohydrates”
Saturated fats support this metabolic flexibility by ‘getting out of the way’ and allowing us to burn carbs, first. In contrast, PUFAs burn first and don’t step aside.
There are a few ways PUFAs “get in the way”:
1) First, they inhibit PDH, a key carb-burning enzyme
When you eat carbs, they are broken down into glucose. This glucose goes through a process called glycolysis, where one glucose molecule is converted into two pyruvate molecules.
At this stage, your body has two choices:
A. Stop at glycolysis, where minimal ATP is produced and unfavorable lactate is generated, which in high levels can contribute to cellular stress and metabolic dysfunction.
B. Go the full route, where pyruvate enters the mitochondria for oxidative phosphorylation: the ideal energy production pathway that produces 30-36 molecules of ATP per molecule of glucose, and beneficial CO2.
To move forward with option B (oxidative phosphorylation, the ideal path), Pyruvate Dehydrogenase (PDH) must convert pyruvate into acetyl‑CoA.
PDH links carbohydrate metabolism to energy production. If PDH is inhibited, glucose cannot be fully oxidized for energy.
PUFA oxidation leads to the accumulation of NADH and a lower NAD+/NADH ratio. PDH requires adequate NAD⁺ to function, so this imbalance directly suppresses PDH activity.
In contrast, saturated fats support PDH by:
>> Favoring FADH₂ production (at Complex II in the ETC)
>> Keeping NAD⁺ levels high
>> Promoting proper pyruvate-to-acetyl-CoA conversion
>> Supporting the Krebs cycle and CO₂ production
Bottom line: Saturated fats make room for carbs to be burned, supporting metabolic flexibility. PUFAs don’t.
2) Second, they Suppress ChREBP, a Carbohydrate Metabolism Regulator
PUFAs also interfere with carbohydrate metabolism by inhibiting ChREBP (Carbohydrate Response Element-Binding Protein) (r), a transcription factor that regulates glucose uptake, glycolysis, and PDH expression.:
When ChREBP is suppressed, PDH activity declines even further, worsening glucose oxidation and impairing metabolic flexibility.
Saturated fats, by contrast, do not suppress ChREBP, allowing for proper glucose metabolism.
3) Third, they can cause oxidative damage to mitochondria
PUFAs are highly unstable and prone to lipid peroxidation: a chain reaction that damages cell membranes, especially within mitochondria.
One of the main targets of this oxidative damage is cardiolipin, a vital mitochondrial phospholipid that stabilizes the electron transport chain and supports the activity of cytochrome c oxidase.
When cardiolipin is oxidized, cytochrome c oxidase becomes dysfunctional, slowing ATP production and compromisingthe body’s ability to burn glucose efficiently.
What do the studies say?
- In a study comparing sunflower oil (PUFA-rich) to heavy cream (SFA-rich), only the cream group showed increased glucose oxidation after a meal + exercise (what we want to see), the sunflower oil group did not. (ref)
- Diets high in soybean oil (PUFA) led to higher blood sugar and insulin levels compared to those with coconut oil (SFA). (ref)
- Another study found that a higher PUFA:SFA ratio increased fat oxidation post-meal, further showing that PUFAs get burned first (r) blocking carb oxidation when it should be prioritized.

You may come across research claiming that PUFAs improve fat oxidation, especially after mixed meals. But this interpretation is fundamentally flawed. Elevated fat oxidation after eating a mixed meal (containing both carbs and fat) is actually a sign that something is off.
If your body is burning fat when it should be burning glucose, then blood sugar stays elevated longer, insulin remains higher, and carbs are more likely to be stored as fat instead of used for energy. In other words, the body isn’t doing what it’s supposed to do: use glucose first, then smoothly shift to fat once insulin drops.
So when studies show that PUFAs “enhance fat oxidation” after meals, that’s not necessarily a good thing. It may reflect a disruption in normal fuel partitioning, not a metabolic advantage.
Some may point to a meta-analysis claiming that PUFAs improve insulin resistance (r), but many of the trials included in that analysis used trans-fat–rich margarines in the so-called “saturated fat” groups. That’s a massive confounder.
Trans fats are well-documented to impair insulin signaling, promote inflammation, and increase disease risk. So when PUFAs are compared against damaging trans fats, the results are misleading and don’t represent a fair comparison.
Key takeaways
PUFAs:
-- Suppress the enzymes and pathways needed to metabolize carbohydrates
-- Promote insulin resistance and metabolic inflexibility
-- Damage mitochondria and slow down energy production
Saturated fats:
-- Support PDH activity
-- Preserve metabolic flexibility
-- Allow your body to efficiently burn carbs and fat
Many stress out about the Randle Cycle (the competition between fats and carbs for oxidation) and fear eating carbs and fats together in a meal, but evidence suggests it’s PUFAs, not saturated fats, that are more likely to disrupt metabolic flexibility and interfere with carbohydrate utilization. Saturated fats are metabolized differently, allow carbs to burn, and have "coexisted" with carbs in human diets for generations without causing widespread insulin resistance, until PUFAs, seed oils, and refined foods entered the picture.
Saturated fat doesn’t get in the way of carb utilization.
In fact, many traditional European cultures have historically remained lean and metabolically healthy despite diets rich in both carbohydrates and fats (ex: French, Swiss, Swedes and Dutch). They consume plenty of carbs (bread, butter, seasonal produce), while their dietary fat sources are highly saturated: butter, dairy and meat. Like how things used to be!

5. Increased Rates of Oxidative Damage Throughout the Body
Fats aren’t just fuel, they also play structural roles in cell membranes, fat stores, and the bloodstream. But again, not all fats behave the same way.
PUFAs are highly unstable due to their multiple double bonds (r,r), making them uniquely vulnerable to oxidation. In contrast, saturated fats are chemically stable because they contain no double bonds. This makes them far more resistant to oxidation and much less likely to generate harmful byproducts inside the body or during cooking.
“When PUFAs were substituted with more saturated fats, lipid peroxidation, proinflammatory gene induction, and hepatic inflammation all declined significantly.” (ref)
It’s important to distinguish:
- Fat oxidation is a healthy, natural process where fats are broken down in the mitochondria to produce energy.
- Lipid oxidation, on the other hand, is a harmful chemical reaction where unstable fats (like PUFAs) break down into lipid oxidation products (LOPs): toxic compounds that serve as biomarkers of oxidative stress, fuel inflammation, and amplify cellular damage (r,r,r,r,r).
LOPs are neurotoxic, cytotoxic, and mutagenic (r,r), meaning they can disrupt enzymes, degrade proteins, damage phospholipids, and impair cell function.
This contributes to metabolic dysfunction and long-term disease. Just like a car crash leaves debris and destruction, oxidized PUFAs leave behind biological wreckage, accelerating aging and disease. In fact, the European Food Safety Authority has set a safety limit for MDA (a type of LOP) at just 30 μg per kg of body weight per day (r), underscoring the serious health risks of these oxidation products.
A powerful metaphor from Chris Masterjohn, PhD, illustrates this idea further:
“PUFAs, in this sense, are like delicate glass… When glass shatters, it invariably leaves behind a mess of dangerous shards… Likewise, when PUFAs shatter they leave behind shards such as MDA, which are capable of damaging proteins, DNA and other structurally and functionally important components of our cells. The best way to avoid shattering glass is to be careful with how one uses, cleans and stores it. Nevertheless, the danger of breaking glass will increase simply by having too much of it around. Likewise, the consumption of excess PUFAs increases oxidative stress even when the oils are fresh and properly cared for.”
PUFA oxidation can happen in two ways:
1. Enzymatic Oxidation
Enzymes like desaturases, COX, and LOX act on PUFAs to form signaling molecules that regulate inflammation and metabolism. While this process is biologically necessary on some level, excess PUFA intake can push the system into a chronically inflamed state. This pathway becomes even more active in poor metabolic conditions or with high exposure to xenoestrogens like plastics and pesticides.
For example, Linoleic Acid (LA, an Omega-6 PUFA) is acted upon by desaturase (D6D) and elongase enzymes to become Arachidonic Acid (AA). AA is then further processed by COX and LOX enzymes to produce inflammatory compounds and oxidized linoleic acid metabolites (OXLAMs). These metabolites, including bioactive compounds like 9-HODE and 13-HODE, act as inflammatory signaling molecules that further amplify oxidative damage throughout the body. Elevated levels of OXLAMs are linked to chronic inflammation, oxidative stress, and the development of metabolic diseases. (r,r)
“Lowering dietary LA significantly reduced the abundance of plasma OXLAMs...These results show that lowering dietary LA can reduce the synthesis and/or accumulation of oxidized LA derivatives that have been implicated in a variety of pathological conditions.” (r)
Even though enzymatic oxidation is regulated, less PUFA = less raw material available for oxidation, reducing the overall burden and risk.
2. Non-enzymatic Oxidation
PUFAs are also highly vulnerable to spontaneous oxidation when exposed to oxygen, heat, light, or free radicals. This triggers lipid peroxidation, generating toxic aldehydes like MDA and 4-HNE.
When the balance of fats in the body shifts toward unsaturated fats (PUFAs) and away from stable saturated fats, oxidation occurs at a much higher rate, leading to more cellular damage, inflammation, and metabolic dysfunction.
And this isn’t just theoretical, it’s well-documented.
Studies consistently show that increasing dietary intake of PUFAs, particularly linoleic acid (LA), leads to higher levels of lipid peroxidation and OXLAMs (ref), while reducing PUFA intake has been shown to lower levels of these harmful metabolites, reduce inflammation and improve metabolic health. (r,r,r,r,r)
Increasing the proportion of PUFAs in the diet and lowering the saturated to unsaturated ratio increases lipid peroxidation. (ref, r,ref, ref, ref)
In fact just 15% of calories from PUFA has been shown to significantly raise oxidative stress markers in healthy men. (r)
In one human study, participants on a PUFA-rich diet had MDA (a major lipid oxidation product) levels 3 to 6 times higher than those on a MUFA-rich diet (r). The difference would have likely been even greater if the control group was eating a diet of traditional animal fats higher in saturated fat.
Similarly, a 12-week low-LA diet decreased plasma LA levels and significantly lowered the levels of four different OXLAMs. (r)
In another study, when subjects reduced their intake of linoleic acid (LA) from 6.8% of their total daily calories to 2.4%, there was a measurable decrease in OXLAMS in their blood plasma. (r) Subjects also experienced a dramatic improvement in headache severity and quality of life.
“Lowering dietary LA significantly reduced the abundance of plasma OXLAMs, and reduced the LA content of multiple circulating lipid fractions that may serve as precursor pools for endogenous OXLAM synthesis. These results show that lowering dietary LA can reduce the synthesis and/or accumulation of oxidized LA derivatives that have been implicated in a variety of pathological conditions.” (r)

Additional research indicates that having less unsaturated fats in your cell membranes can reduce susceptibility to lipid peroxidation (r). Even a 5% increase in PUFA content in cell membranes has been shown to result in 16 times more peroxidative damage, a pattern seen in both human and animal studies across numerous conditions (r,r,r,r,r).
Where does lipid oxidation occur?
Lipid peroxidation isn’t limited to tissues and the bloodstream, it can also occur during digestion. (r,r) And the more PUFAs present in a meal, the more oxidation occurs, generating harmful byproducts that your body absorbs. (r)
Research suggests that the acidic environment of the stomach can trigger and accelerate this oxidation process (r), especially when pro-oxidant compounds are present, leading to even more lipid peroxides than were in the food to begin with. A
Even before digestion, oxidation can start during cooking. High-heat cooking of PUFA-rich foods creates toxic aldehydeslike MDA and 4-HNE (r,r,r). One study found that the more PUFA in raw meat, the more oxidation products formedwhen cooked (r). Similarly, eggs higher in PUFA were found to oxidize more easily (r, r. r) reducing their shelf life, and compromising their nutritional value, flavor, and aroma. (r)
So when you cook PUFA-rich foods, you’re not just reducing quality, you’re eating pre-oxidized fats that are ready to cause damage once ingested.
Limiting excessive PUFA intake and favoring fats that are higher in more stable, saturated fats can help reduce oxidative damage, support better metabolic health, lower inflammation, and reduce your chronic disease risk (r)
That’s because the damage caused by lipid peroxidation isn’t isolated, it’s systemic.
Here are just a few examples of how the products of PUFA oxidation can cause damage throughout the body:
- Atherosclerotic plaque formation: Lipid peroxides and aldehydes play a key role in the formation of plaque in arteries, increasing the risk of cardiovascular disease. (r,r)
- Arterial damage: Diets high in PUFAs are associated with increased damage to arterial walls, raising the risk of hypertension and heart disease. (r)
- Age-related macular degeneration (AMD): High PUFA intake is linked to a higher risk of AMD (r), a condition where the macula of the retina becomes damaged, impairing central vision and color perception.
- Kidney damage: Lipid peroxidation in the kidneys reduces antioxidant defenses, increases inflammation, and leads to further kidney damage. (r,r)
- Alzheimer’s disease: Individuals with Alzheimer’s disease show higher levels of lipid peroxidation products in their brains, contributing to neurodegeneration. (r,r,r)
- Brain damage: A 2020 mouse study found that diets high in soybean oil (rich in LA) dysregulated over 100 genes related to inflammation, neuroendocrine function, insulin signaling, and neurological health (r, r, r). These changes were linked to obesity, diabetes, and neurological conditions like autism, Alzheimer’s, anxiety, and depression. Excess dietary LA also increases brain susceptibility to inflammation. (r)

- Cancer risk: A recent study (r) has identified a key mechanism by which linoleic acid (LA) drives the growth and spread of aggressive cancers like triple-negative breast cancer, by activating the enzyme FABP5, which is linked to poor prognosis across many cancer types. Given LA’s role in promoting inflammation and its dramatic rise in consumption since the 1950s, it’s increasingly clear that PUFAs may not only fuel cancer progression but also play a role in its root cause. These oxidation products cause damage that can lead to chronic disease and cancer.
“we propose that the major bioactive product of oxidative degradation of polyunsaturated fatty acids (PUFAs), the reactive aldehyde 4-hydroxynonenal (4-HNE), which is also considered a second messenger of free radicals, may be the key pathogenic factor linking diabetes and cancer.” (ref)
- Liver Damage: When PUFAs, especially linoleic acid, accumulate in liver cells, they become highly prone to oxidation. This oxidative stress triggers inflammation and immune responses, causing cellular damage and fibrosis - the scarring process that impairs liver function over time. In fact, higher PUFA intakes increase the risk of nonalcoholic fatty liver disease (NAFLD) (r,r) and its progression to more severe liver conditions like NASH (r), whereas saturated fats offer protective effects against these issues. (r, r, r, r, r) Some experimental and epidemiological studies have shown that linoleic acid is required for the development of alcoholic liver damage (ref). In contrast, saturated fats and cholesterol have been shown to protect against liver fibrosis. (r, r, r, r, r)
In one study 6-month dietary intervention to reduce LA intake completely reversed fatty liver disease in all 24 patients, along with significant drops in lipid oxidation products (r). Truly empowering results!

- Accelerated Skin Aging and Damage: A PUFA rich diet increases the risk of sun burn (r,r,r,r,r), since your dietary fat choices impact the composition of the types of fat in your skin. (ref)
Our skin naturally contains some amount of PUFAs, as they are naturally incorporated into the skin’s fats. However, a higher PUFA diet will shift the skin’s lipid profile toward more unsaturated fats. (r)
This increase in PUFAs raises the risk of oxidation, which becomes especially concerning when the skin is exposed to ultraviolet (UV) rays from the sun. Under UV exposure, excess PUFAs are more likely to undergo oxidation, creating harmful free radicals that damage skin cells. This oxidative damage accelerates aging, triggers inflammation, and increases the risk of skin cancer. (r)
Studies support this, showing that UV exposure oxidizes skin lipids (especially if rich in PUFAs), leading to erythema (redness) and significant damage. A 1995 study in the Journal of Investigative Dermatology found elevated lipid peroxide levels in UV-exposed skin with higher PUFA content, suggesting that dietary unsaturated fats can amplify this effect. (r)

In contrast, saturated fats are more stable and less prone to oxidation, meaning they don’t produce the same harmful effects when exposed to sunlight.
Additionally, a higher intake of linoleic acid has been linked to an increased risk of skin cancers. (r) In fact, eliminating seed oils from the diet has been associated with a dramatic reduction in the risk of UV-induced sunburn (r) since skin susceptibility to UV damage is directly influenced by the amount of LA in the diet.(r,r)
Dr. Ray Peat elaborates on the detrimental effects of excess unsaturated fats in the diet -- “In the l960s, Hartroft and Porta gave an elegant argument for decreasing the ratio of unsaturated oil to saturated oil in the diet (and thus in the tissues). They showed that the “age pigment” is produced in proportion to the ratio of oxidants to antioxidants, multiplied by the ratio of unsaturated oils to saturated oils. More recently, a variety of studies have demonstrated that ultraviolet light induces peroxidation in unsaturated fats, but not saturated fats, and that this occurs in the skin as well as in vitro. Rabbit experiments, and studies of humans, showed that the amount of unsaturated oil in the diet strongly affects the rate at which aged, wrinkled skin develops. The unsaturated fat in the skin is a major target for the aging and carcinogenic effects of ultraviolet light.”
Another compelling animal study illustrates this point (r): rabbits fed a diet high in PUFAs (10% corn oil) exhibited significantly more skin damage after UV exposure compared to rabbits on a diet high in saturated fats (10% coconut oil). The increased oxidation of PUFAs in the skin cells led to more sunburn and accelerated aging, evidenced by more wrinkled skin.
The composition of your skin reflects what you eat, and the oxidation of PUFAs under UV exposure highlights the importance of paying attention to your fat intake. Reducing PUFA-rich seed oils in the diet and prioritizing more stable fats can help protect the skin from sun damage, aging, and even skin cancer.
Choose fats that don’t break you down.
By being intentional with your fat choices, you reduce the raw material that breaks down into harmful byproducts, whether during cooking or inside your body.
Saturated fats are stable and more resistant to oxidation (r). PUFAs are unstable, easily oxidized, and contribute to a wide range of chronic diseases when consumed in excess.
6. Increase Cardiovascular Disease Risk
While mainstream nutrition often brands PUFAs like particularly linoleic acid (LA) as “heart‑healthy,” the evidence doesn’t fully support that narrative.
In fact, re-analyses of the original landmark trials show that replacing saturated fat with linoleic acid-rich oils lowers cholesterol but does not consistently reduce deaths from coronary heart disease or overall mortality:
“Available evidence from randomized controlled trials shows that replacement of saturated fat in the diet with linoleic acid effectively lowers serum cholesterol but does not support the hypothesis that this translates to a lower risk of death from coronary heart disease or all causes. Findings from the Minnesota Coronary Experiment add to growing evidence that incomplete publication has contributed to overestimation of the benefits of replacing saturated fat with vegetable oils rich in linoleic acid.” (r)
While LDL is often labeled “bad cholesterol,” it’s not inherently harmful. The real danger comes from the oxidation of linoleic acid within LDL particles (r,r), which makes them unstable and more likely to contribute to arterial plaque buildup. (r,r)
Research in the 1980s and ‘90s revealed that changes in total cholesterol or LDL cholesterol levels were poor predictors of who developed heart disease. A much better predictor was the susceptibility of LDL particles to oxidation (r,r). And the most likely fatty acid to oxidize in LDL particles is linoleic acid (r,r).
Oxidized LDL refers to LDL particles whose components (especially linoleic acid in their phospholipids) have undergone oxidative modification. Because LA is the most abundant and oxidation-prone fatty acid in LDL, its breakdown products dominate oxidized LDL (r).
Elevated oxidized LDL is one of the strongest biomarkers we have for cardiovascular risk. In fact, new research from 2021 suggests that atherosclerosis might not even be possible without oxidized LDL cholesterol. (r)
Supporting evidence:
> Patients with cardiovascular disease have higher levels of linoleic acid in their blood (r), and the severity of atherosclerosis correlates with higher concentrations of oxidized linoleic acid in arterial plaques. (r)
> Autopsy studies confirm that linoleic acid is the predominant fat in atherosclerotic plaques (r), linking seed oils and high-PUFA diets directly to cardiovascular risk. (r,r)
> The more severe the patient’s heart disease was before their death, the more oxidized linoleic acid was found in their plaque. (r)
> PUFA oxidation products like peroxides and aldehydes (such as HNE and MDA) are key factors in atherosclerotic plaque formation. (r, r)
Simply put, the more linoleic acid consumed, the higher the risk that it will integrate into blood lipoproteins, increasing their susceptibility to oxidation (r), a key trigger for heart disease.
7. Disrupt Gut Health
More and more people today are struggling with gut issues and food intolerances, problems our ancestors rarely faced. One reason? PUFAs. These fats can significantly disrupt gut health (r) by damaging intestinal structure and driving oxidative stress.
Linoleic acid, the dominant omega-6 PUFA in the modern diet, has been shown to:
> Decrease intestinal epithelial barrier function (r), weakening the gut’s defense against harmful substances.
> Alter the gut microbiome leading to gut dysbiosis (r,r), an imbalance in gut bacteria that contributes to inflammation and digestive issues.
> Harm intestinal wall integrity by reducing the height and width of villi (r), the small, finger-like projections in the gut that are essential for nutrient absorption.
> Increase tight junction permeability (r, r) by downregulating tight junction protein expression (r). Tight junctions are critical for maintaining a healthy intestinal barrier with selective permeability; when they weaken, endotoxins and other harmful compounds can leak into the bloodstream, driving systemic inflammation.
> Promoting gut inflammation (r), further weakening the gut barrier and increasing vulnerability to chronic digestive disorders.
These effects increase susceptibility to inflammatory bowel diseases (IBD), and research suggests that PUFAs may play a direct role in their development. (r, r)
In one study, they exposed human intestinal cells to lipid hydroperoxides (oxidized lipids) and observed increased apoptosis, indicating that lipid peroxidation products can damage the protective lining of your gut, making it less effective at acting as a barrier and potentially leading to issues like inflammation or leaky gut.
One study exposed human intestinal cells to lipid hydroperoxides (oxidized fats) and found increased apoptosis (ref), showing that lipid peroxidation products can damage the protective gut lining. This damage weakens the barrier’s ability to filter harmful substances, opening the door to inflammation and “leaky gut.”
A striking visual from a research study (r) demonstrates the point above about how PUFAs downregulate mRNA expression of both tight junction proteins and their adaptor proteins:
“We demonstrate that USF (corn oil/linoleic acid) by itself results in dysregulation of intestinal TJ integrity leading to increased gut permeability” (ref)
Again, tight junction proteins act as gatekeepers, regulating what passes through the intestinal wall. When expression is downregulated, the barrier weakens, allowing inflammatory compounds to enter circulation, fueling gut dysbiosis, food intolerances, and systemic inflammation.

Another study investigated how different fatty acids affect the host-microbe relationship in the gut:
“The corn oil diet, rich in Omega 6 polyunsaturated fatty acids, increased the potential for pathobiont survival and invasion in an inflamed, oxidized and damaged gut, while saturated fatty acids promoted compensatory inflammatory responses involved in tissue healing.” (ref)

In contrast, saturated fats are protective (r, r, r). They reduce oxidation, support gut barrier integrity, and can even bind to endotoxins to help neutralize them. Prioritizing fats that are higher in saturated fats and lower in PUFAs may be one of the most important steps in protecting gut health and reducing chronic inflammation.
8. Increase Stress
Unsaturated fats have been shown to have a distinct impact on our stress response. While the body relies on certain mechanisms to handle stress, it turns out that the type of fats we consume can either support or hinder these processes.
Corticotropin-releasing hormone (CRH) is the body’s primary external stress signal. When you experience stress, the hypothalamus releases CRH, which prompts the pituitary gland to release adrenocorticotropic hormone (ACTH). This, in turn, signals the adrenal glands to produce cortisol, the main stress hormone. ACTH amplifies the stress response by driving cortisol production, creating an internal cascade of stress signals in the body (r).
Interestingly, research shows that unsaturated fats can increase the production of both cortisol and aldosterone (r). Aldosterone plays a role in regulating fluid balance, but excess levels can lead to fluid retention, inflammation, and fibrosis, contributing to high blood pressure, damage to blood vessels, and heart issues.
This altered stress response is likely driven by lipid oxidation products. When PUFAs like linoleic acid undergo oxidation, they can form compounds such as EKODE, which has been shown to stimulate steroidogenesis, including cortisol and aldosterone production. (r, r)
In contrast, saturated fats are less prone to oxidation and thus do not produce these types of lipid oxidation products, which may be why they do not contribute to the same increase in cortisol and aldosterone production. (r, r)
One study highlighted that while CRH and ACTH drive stress hormone release, unsaturated fatty acids actually exacerbate this process, while saturated fats blunt the release of ACTH and cortisol in response to CRH (r).
Several other studies point to the potential for PUFAs to contribute to chronic stress or make stress-related conditions worse, while saturated fats seem to offer a protective effect (r). By reducing the overproduction of stress hormones like cortisol and aldosterone, saturated fats may help mitigate the long-term damage that excess stress hormones can cause, including tissue damage in organs like the heart and kidneys. (r)
These findings demonstrate yet another example of how the body processes unsaturated and saturated fats differently. While PUFAs may heighten stress and its associated risks, saturated fats may play a supportive role in helping the body manage stress more calmly. This is an important consideration in managing both short-term and long-term health, as chronic stress is a well-known contributor to various health issues, including heart disease and metabolic dysfunction.
9. Contribute to Hormonal Imbalances (in Women AND Men)
PUFAs can have a significant impact on estrogen metabolism in the body. One of the key ways they do this is by decreasing estrogen detoxification in the liver, which can contribute to estrogen imbalances (r). The liver is crucial for processing and eliminating excess estrogens, but when PUFAs are present in high amounts, they can impair this function, leading to a buildup of estrogen in the system. (r)
Estrogens are stored in fat tissue but detoxified via a process called 2-hydroxylation, which makes estrogen molecules more water-soluble for easier excretion. “[H]igh intake of the linoleic acid and arachidonic acid inhibits the detoxification of oestrogens by 2-hydroxylation and increases 16-alpha-hydrolxyation, resulting in metabolites that can undergo redox cycling and generate hydroxyl radicals.”(r)
In addition to impairing estrogen detoxification, PUFAs can amplify existing estrogen imbalances (r,r). PUFAs are considered estrogenic since can increase aromatase activity (r,r), which is the enzyme that converts androgens (like testosterone) into estrogens, further tipping the hormonal balance toward estrogen dominance.
Estrogen dominance occurs when there is an excess of estrogen in the body relative to other sex hormones, especially progesterone in women and testosterone in men. Interestingly, many people in the modern world may unknowingly be in a state of estrogen dominance. Many people today may unknowingly be in this state, partly due to the misconception that low serum estrogen means low overall estrogen. Serum estrogen reflects only what is circulating in the blood, not what’s stored in body tissues (r,r).
These disruptions become even more concerning when combined with modern environmental exposures. Pervasive chemicals like microplastics and pesticides can mimic or interfere with estrogen’s natural actions, further destabilizing hormonal regulation. Layer these on top of PUFA-induced effects, and you create a perfect storm for estrogen-related health issues, from fertility challenges to hormone-sensitive cancers. Simply put, we do not need more estrogen-mimicking compounds in our lives.
10. Changes the Types of Fat Inside of You, Which Changes How You Function
This big fat shift didn’t just change what’s on our plates — it changed us. Dietary fat isn’t just fuel; it becomes a literal part of our bodies.
The fats you eat impacts the types of fat inside of you. (ref, ref, ref)
Over the last century, the fatty acid makeup of the human body has shifted dramatically. Compared to our great-grandparents, we’re less saturated (r) and more unsaturated, meaning our tissues are now more prone to oxidation (r,r).
This change directly reflects what we eat and how our food is produced.
The first solid data on body fat composition came in 1943 (r): 37% saturated fat, 55% monounsaturated fat, and 10% PUFA. Stearic acid, a saturated fat, and linoleic acid, an Omega 6 PUFA, were each at ~8%.
By 1991, those numbers had shifted dramatically (r): 24% saturated fat, 55% monounsaturated fat, and 21% PUFA. Stearic acid dropped down to 2.9% and linoleic acid rose to 17.2%
That’s the opposite trend we want, and those numbers were from the 1990s. A 2015 review confirmed that PUFA levels in body fat, especially linoleic acid (LA), have continued to rise, increasing 136% over the last 50 years in parallel with dietary LA intake (r).
“LA has increased by 136% over the last half century and that this increase is highly correlated with an increase in dietary LA intake over the same period of time…Our findings suggest that adipose tissue LA concentration is strongly correlated with dietary LA intake.” (r)

Because something goes up only if something else goes down, our saturated fat levels have fallen (r), moving us further from our natural design.
The American diet flipped from high-saturated to high-unsaturated fats—and our bodies changed with it.
Why does this matter?
Saturated and unsaturated fats have different chemical structures, and shifting the balance changes how your body works on a cellular level (as discussed in a previous section). When your body burns stored fat for energy, it pulls whatever you’ve built into your fat cells. The more PUFAs you’ve stored, the more unclean fuel you burn, negatively impacting energy production.
This shift is so profound it’s affecting the next generation from birth. A mother’s diet influences the fatty acid composition of her breast milk (r, r). Diets high in PUFAs increase PUFA levels and reduce beneficial saturated fats in breast milk (r,r,r). Historically, LA made up about 5% of breast milk fat; today, it’s often 15–25% (r), reflecting changes in both maternal diet and body fat stores. This shift has been linked to concerning outcomes in developing children. Higher maternal PUFA intake is associated with an increased risk of delayed psychomotor and mental development (r), autism (r,r), allergy development (r), lower cognitive scores (r,r,r), and childhood overweight and obesity (r,r,r).

Fat becomes part of your structures.
Fat isn’t just stored in adipose tissue, it’s a key building block in your organs, tissues, and cells.
Take phospholipids, for example: fat-based molecules that form cell membranes. Their two fatty acid “tails” can be made from different types of dietary fats, and what you eat changes their composition (ref). In mitochondria, the fatty acid profile of these phospholipids mirrors the profile of your diet (ref).


Change the fats, and you change the membrane’s structure and function (ref).
More saturated fats create membranes that are tighter, more resilient, and stable (ref). More PUFAs make membranes overly fluid, fragile, and prone to oxidative damage (ref, ref).

Image from [ref]
Cardiac studies show that altering fatty acid composition in membrane lipids can impair heart function:
“The above results suggest that alterations in FA composition in the heart may contribute to deterioration of heart function. A possible mechanism of this phenomenon is the alteration of sphingolipids and phospholipids in the fatty acid profile, which may change the physical properties of these lipids.” (ref)
In mitochondria, stable, organized membranes are essential for optimal ATP and CO₂ production.
While membranes aren’t meant to be fully saturated and rigid, the right balance of saturated and unsaturated fats supports efficient energy production. Poor membrane structure means poor energy production and impaired cell function.
Research actually shows that a higher degree of fatty acid saturation in the body correlates with resistance to several types of cancer (ref,ref).
So when we say “we become what we eat”, it’s not just poetic, it’s literal. And today, we’re rebuilding the architecture of our biology out of unstable materials.
The Saturated to Unsaturated Fat Ratio
The American metabolism isn’t just slowing down, it’s being actively suppressed by the types of fats that dominate the modern diet. High PUFA intake can downregulate energy production, impair thyroid function, and alter the way your body burns fuel — all of which lower metabolic rate and encourage fat storage.
Over the last century, we’ve shifted toward more unsaturated fats and fewer saturated fats.
The SFA:UFA ratio refers to the proportion of saturated fatty acids (SFA) to unsaturated fatty acids (UFA) in your diet.Even if your total fat intake stays the same, a high-PUFA, low-SFA diet sends different metabolic signals than one rich in saturated fats. Over time, this can change your body fat composition, affect cell membrane function, and even influence how well you digest and tolerate certain foods.
Improving this ratio isn’t about eating more total fat, it’s about changing the balance toward fats that support metabolic health.
To really drive this point home, let’s compare two examples - both of which contain the same amount of total dietary fat intake - 60 grams. (Note: These two lists show only the fat sources in each example, they’re not meant to represent a complete day of eating with additional carbs and protein).
So with the example on the left, the SFA:UFA > 1 (more saturated than unsaturated), and it consists of 3 corn & soy free Low PUFA Angel Acres eggs, 2 oz raw cheese, 1 Tbsp butter, ground beef, and some cottage cheese.
Now compare this to a different 60 grams of fat, but more unsaturated fat rich sources. Where the SFA/UFA ratio is < 1. Fried chicken, oat milk, margarine, French fries and potato chips - fried in vegetable oils.
Both examples have the same total fat, but the biochemical structure of those fats matters. Different SFA:UFA ratios influence the NAD⁺/NADH ratio and the redox state of cells throughout the body, sending very different metabolic signals.

You don’t have to track your SFA:UFA ratio perfectly, but it’s important to understand that your cumulative dietary fat sources impact carbohydrate metabolism, oxidative damage, inflammation, and fat storage.
Shifting the balance toward more saturated fats and fewer PUFAs can help restore efficient energy production, protect against oxidative stress, and support a healthier metabolism.
Aren’t PUFAs essential?
Mainstream nutrition guidelines label both omega-6 and omega-3 fatty acids as “essential” because our bodies can’t make them on their own. But how much do we actually need?
Many researchers now suggest that the requirement for PUFAs has been significantly overestimated. (r,r,r,r,r) Newer estimates put true physiological needs 5–10 times lower than previously believed (r) (and currently still promoted).
In other words, you don’t need to go out of your way to add them to your diet, simply eating whole, unprocessed foods naturally provide enough to meet your body’s minimal requirements. Even animal fats contain small amounts of PUFAs, which help cover these small “essential” needs with ease. (There are some scientists that argue these aren’t actually essential, but that’s a story for another day!)
Keep in mind that Omega-3s are PUFAs, too. They are also unstable and prone to oxidation (r), which can cause problems when consumed in excess (r).
I am not saying you shouldn’t consume Omega-3s. I think animal-based Omega-3s (like DHA) can be beneficial when consumed in natural, non-fortified amounts.
Plant-based Omega 3’s like ALA is a different story. For instance, studies show that increasing Omega-3s (through flax feeding, boosting plant-based Omega 3 levels) in chicken doesn’t reduce inflammation but instead makes the meat more susceptible to oxidation, compromising its quality and stability. (r,r) The current Omega-3 obsession, fueled by marketing, pharmaceutical interests, and processed food fortification, has distorted our understanding of healthy fats. Consuming very high levels is neither normal nor beneficial over the long term.
The end goal isn’t to eliminate PUFAs entirely, as that would be both impractical and unnecessary. However, returning to more ancestrally consistent levels of PUFAs, similar to what our ancestors naturally consumed, seems like a healthier approach. Nature already designed a balanced fatty acid profile; rather than artificially manipulating it, the better strategy is to reduce overall PUFA intake and focus on stable, nutrient-rich fats.
Today, the average person gets about 15% of daily calories from PUFAs, far higher than the levels our ancestors consumed. This increase is largely due to the rise of industrial seed oils and changes in livestock feed, which have raised PUFA content in pork, chicken, and other animal products.
While PUFA deficiencies are virtually unheard of, chronic overconsumption is becoming a major driver of metabolic dysfunction and chronic disease.
What to do?!?
After a century of manipulating food to fit a false health narrative, we need to face the truth: the shift in our food’s fatty acid profile is damaging our health.
We now consume diets higher in unsaturated fats and lower in saturated fats. And it’s not working.
So, what can you actually do?
Step one is understand the problem.
PUFA consumption has gone up.
And when something goes up, something else has to go down....
Unfortunately, that “something else” has been health-promoting, metabolism-boosting saturated fats—especially stearic acid. So not only are we consuming more unstable unsaturated fats, but we’re also missing out on critical fats like stearic acid that support metabolic health.
Instead of nourishing our bodies with the stable fats we evolved with, we’ve flooded our systems with unstable plant-based polyunsaturated fats thanks to decades of flawed advice promoting PUFAs as “heart healthy.”
The reality? Excess PUFA intake has been linked to metabolic decline, lipid peroxidation (aka internal rust), inflammation, insulin resistance, hormone imbalances, and a host of chronic conditions from diabetes to cancer.
Plant-based PUFA-rich fats have no ancestral role in our diets.
But animal fats higher in saturated fats have always been there, supporting us across generations.
Why does this matter? Because the balance of saturated and unsaturated fats in our diet directly impacts cell signaling, gene expression, and metabolic function.
Step two is to take action.
It’s time to return to the traditional, nutrient-dense fats that nourished humans for generations.
Be mindful of both how much fat you’re eating and the type of fat you are consuming.
And when it comes to lowering your PUFA intake, consistency is key. If you keep reaching for high-LA foods, you’re simply refilling your fat stores with the same unstable compounds, keeping yourself stuck on the metabolic hamster wheel.
Remember: your body can’t make these plant-based PUFAs. The only way they end up in your system is through your diet.
A great place to start is with an app like Cronometer. It helps you track your fat intake so you can gather real data and see where improvements can be made, especially when it comes to balancing your saturated to unsaturated fat ratio.
Because of how profoundly dietary fats impact your health, I’d argue that prioritizing the quality of your fat sources, and choosing low-PUFA options whenever possible, is one of the most important food decisions you can make.
That said, zero PUFA isn’t the goal, nor is it realistic. Tiny amounts of PUFA occur naturally in animal fats in biologically appropriate ratios—and that’s totally fine.
Zero PUFA isn’t realistic or necessary. Small amounts naturally occur in animal fats in biologically appropriate ratios, and that’s fine. The problem is excess from modern refined plant oils and PUFA-rich conventionally raised pork and chicken. Reducing these concentrated sources is key.
Limiting PUFAs and prioritizing stable, saturated fats is a simple but powerful shift, one that supports less damage, better energy metabolism, and long-term health resilience.
Want to learn more about the best and worst food for a low PUFA diet? Check out this blog post.
The LowPs™ Difference
After a century of manipulating our food to fit a false health narrative, the shift in our food’s fatty acid profile is taking a serious toll on our health. It’s time to return to the traditional, nutrient-dense fats that nourished humans for generations.
Stearic acid and other health-promoting saturated fats are naturally found in grass-fed beef, tallow, butter, pasture-raised eggs, and chicken. But here’s something most people don’t realize: what an animal eats changes the fat in its meat, milk, and eggs, and that’s the fat you eat.
Today’s livestock are fed very differently than they were 100 years ago, and that shift has altered the fat profile of the food on our plates. More PUFAs in livestock feed means more PUFAs (and fewer saturated fats) in the final product.

Simply put: better food for them means better health for you.
So why did we build Nourish Food Club?
Five years ago, we couldn’t find traditional animal fat sources anywhere, even from farms labeled regenerative, pasture-raised, or organic. Most were still feeding corn, soy, or distillers grains, which push PUFA levels higher.
We got fed up with the lack of truly trustworthy options, so we built our own supply chain from the ground up.
At Nourish, we don’t just care about what’s on your plate, we care about the entire process that gets it there.
- - Our beef, lamb, and dairy sheep are 100% grass-fed and rotationally grazed on pasture.
- - Our chickens, pigs, and dairy goats are fed a custom, low-PUFA, corn- and soy-free diet designed to support metabolic health.
Just like our great-great grandparents enjoyed and drastically different from what’s widely available today.
Our goal isn’t to eliminate every trace of PUFA, that’s neither realistic nor necessary. But we can restore a healthier fat balance that mirrors what food used to be.
Modern diets are sending the wrong metabolic signals.
We’re here to change that.
Let’s rebuild our health by rebuilding how we farm.
This is food the way it was meant to be, before industrial agriculture PUFA’d everything.